DOSINg & Administration
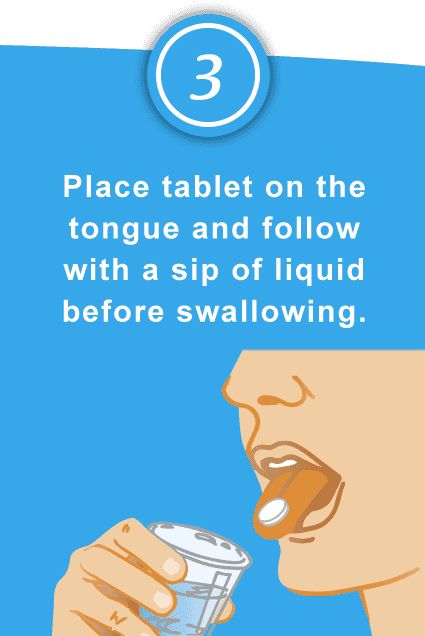
Taking SPRITAM is as easy as…



SPRITAM tablet 11. Boudriau S, Harvey C, Massicotte J, et al. Randomized Comparative bioavailability of a nocel three-dimensional printed fast-melt formulation of levetiracetam following the administration of a single 1000-mg dose to healthy human volunteers under fasting and fed conditions, Drugs R D. 2016;16(2):229–238.:
-
Should be taken whole
-
Should be swallowed intact
-
Can be taken with or without food
Fasting vs Fed
In a clinical study of 33 healthy volunteers, consuming food (a high-fat, high-calorie breakfast) decreased the peak plasma concentration (Cmax) by 36% and delayed the time to peak plasma concentration (Tmax) by 3.5 hours. However, it did not affect the extent of absorption. 11. Boudriau S, Harvey C, Massicotte J, et al. Randomized Comparative bioavailability of a nocel three-dimensional printed fast-melt formulation of levetiracetam following the administration of a single 1000-mg dose to healthy human volunteers under fasting and fed conditions, Drugs R D. 2016;16(2):229–238.
Refer to the Full Prescribing Information for complete dosing recommendations 11. Boudriau S, Harvey C, Massicotte J, et al. Randomized Comparative bioavailability of a nocel three-dimensional printed fast-melt formulation of levetiracetam following the administration of a single 1000-mg dose to healthy human volunteers under fasting and fed conditions, Drugs R D. 2016;16(2):229–238.:
Partial-Onset Seizures
| Patient Age and Weight | Initiation | Titration | Maximum Recommended Dose |
|---|---|---|---|
| Adults and pediatric patients 4 years and older (>40 kg) | 1,000 mg/day (500 mg twice daily) | Increments of 1,000 mg/day every 2 weeks (500 mg twice daily) | 3,000 mg/day (1,500 mg twice daily) |
| Pediatric patients 4 years and older (20 kg to 40 kg) | 500 mg/day (250 mg twice daily) | Increments of 500 mg/day every 2 weeks (250 mg twice daily) | 1,500 mg/day (750 mg twice daily) |
Myoclonic Seizures
| Patient Age | Initiation | Titration | Maximum Recommended Dose |
|---|---|---|---|
| Patients 12 years and older with juvenile myoclonic epilepsy | 1,000 mg/day (500 mg twice daily) | Increments of 1,000 mg/day every 2 weeks (500 mg twice daily) | 3,000 mg/day (1,500 mg twice daily) |
Primary Generalized Tonic-Clonic Seizures
| Patient Age and Weight | Initiation | Titration | Maximum Recommended Dose |
|---|---|---|---|
| Adults and pediatric patients 6 years and older (>40 kg) | 1,000 mg/day (500 mg twice daily) | Increments of 1,000 mg/day every 2 weeks (500 mg twice daily) | 3,000 mg/day (1,500 mg twice daily) |
| Patients 6 years and older (20 kg to 40 kg) | 500 mg/day (250 mg twice daily) | Increments of 500 mg/day every 2 weeks (250 mg twice daily) | 1,500 mg/day (750 mg twice daily) |
Switching to SPRITAM from a conventional form of immediate-release (IR) levetiracetam
All 4 strengths of SPRITAM are designed to deliver an equal amount of levetiracetam as other marketed dosage forms based on pharmacokinetic bridging studies.11. Boudriau S, Harvey C, Massicotte J, et al. Randomized Comparative bioavailability of a nocel three-dimensional printed fast-melt formulation of levetiracetam following the administration of a single 1000-mg dose to healthy human volunteers under fasting and fed conditions, Drugs R D. 2016;16(2):229–238.
For example:
